Neurobiology of Memory
Leaders
Our research group is interested in the neurobiological mechanisms that lead to the earliest and most characteristic symptom of Alzheimer’s disease (AD), impairment of recent memory. Since the earliest pathological changes of AD cannot be studied in a human post mortem brain (except in rare genetically determined forms of the disease), we will use as a disease model transgenic mice that carry human familial AD linked mutations found in a Swedish (APPswe) and a Finnish (PSEN1dE9) family. With the help of these mice we can follow the development of pathophysiological changes at the cellular level from their onset. The neuropathology we will examine by using various histochemical stainings and with fluorescence and confocal microscopy.
We assess the disease-associated symptoms in mice by various behavioral tests, mostly those drawing on different memory processes. Furthermore, we study the brain function by electrophysiological recordings. We monitor mouse sleep-wake rhythm and sleep-related epileptiform discharges with video-EEG. In addition, we assess the communication between different brain regions during a memory task and the following sleep epoch by recording brain oscillations and neuronal activity with implanted deep electrodes. In collaboration with prof. Olli Gröhn’s group (AIVI) we conduct simultaneous EEG and functional MRI recordings.
We also carry out preclinical studies investigating the impact of new drugs or other treatments on the AD disease process and memory impairment. Our group was the first one to demonstrate that the latest AD drug memantine has beneficial memory effects in an AD mouse model.
Ongoing projects
- Contribution of epileptic spiking to fluctuating memory impairment in AD
- Drug treatments against sleep-related epileptiform spiking in AD
- Role of meningeal lymphatic vessels in early amyloid pathology (A Finnish consortium)
- Early intra- and extracellular amyloid pathology (A Nordic consortium)
- Contribution of type 2 diabetes to tau pathology in AD
-
Our primary disease model is a transgenic mouse model carrying Swedish APP and Finnish PSEN1dE9 human gene mutations that cause rare familial forms of AD.
Among numerous AD model mice this line is different in that amyloid plaque pathology starts early (~3 months of age) and is fully developed before memory impairment becomes evident (~12 months of age). This order of events corresponds to present understanding of the disease course.
Mouse neurological and neuropsychological testing.
Through a broad behavioral test battery we can fully characterize the phenotype of AD mouse models and assess the effect of novel therapeutic approaches.
 Grid hanging is a simple test for static muscle force.
Grid hanging is a simple test for static muscle force. Accelerating rotarod measures muscle coordination and balance.
Accelerating rotarod measures muscle coordination and balance.
Morris swim navigation task is a classic test for spatial memory. We use the Ethovision video analysis system to track the swim path and the mouse search pattern to find the submerged resting platform.
 Novel object recognition task is a common nonspatial memory test. The success of the test depends heavily on the level of anxiety of the mouse.
Novel object recognition task is a common nonspatial memory test. The success of the test depends heavily on the level of anxiety of the mouse.In vivo electrophysiology
By using multiple bi- or tripolar implanted microelectrodes into areas of interest, we can assess long-term changes in local electrical activity at any brain site, as well as to study the cross-talk between these brain sites. Recordings are combined with video monitoring, also during a memory task. We also combine EEG recordings with BOLD-fMRI in mice.
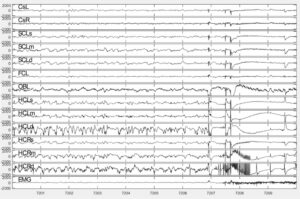 Epileptic seizure in an APP/PS1 mouse starts focally in the right hippocampus (channel HCRd) and gradually becomes generalized.
Epileptic seizure in an APP/PS1 mouse starts focally in the right hippocampus (channel HCRd) and gradually becomes generalized.Gene transfer
 By using viral vectors we can transduce production of any protein of interest locally in the brain to study the molecular interactions behind the memory problems or to reverse the pathological process in a treatment attempt.
By using viral vectors we can transduce production of any protein of interest locally in the brain to study the molecular interactions behind the memory problems or to reverse the pathological process in a treatment attempt.Histopathology
At the end of any study, the mouse brain is carefully examined for hallmarks of AD pathology (amyloid deposit, tau phosphorylation, neuroinflammation) and other processes of interest.
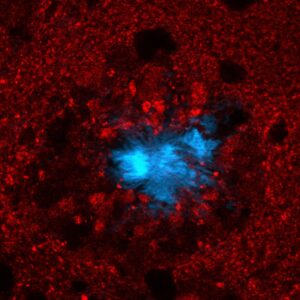
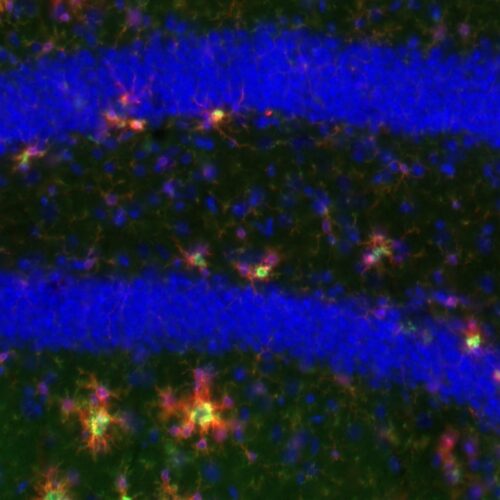
An amyloid plaque stained with DAPI (blue) to reveal the fibrillar plaque core and with vGlut1 (red) to show glutamatergic axon tearminals around the plaque. The axon terminal in contact with the plaque are deformed and enlarged. Further, around the plaque there is a zone devoid of any glutamatergic terminals.
-
- We have carefully characterized the brain distribution of amyloid accumulation in a typical APP/PS1 mouse model and demonstrated impaired hippocampal-dependent spatial memory but intact striatal-dependent response habit learning in APP/PS1 mice
- We were the first to show that amyloid accumulation is faster in female than in male APP/PS1 transgenic mice
- We were the first to show that the latest AD drug memantine has beneficial cognitive effects in a transgenic AD mouse model
- We have demonstrated that manipulation of dietary lipids has a major impact on cognitive functions in APP/PS1 mice
- We were the first to show that hippocampal CA3 area becomes hyperactive in aged rats and perturbs the balance in the hippocampal neural network, a finding that has recently been confirmed in MCI patients
- We were the second lab in the world to report that APP transgenic mice display frequent seizures and the only one that has conducted a systematic study on the in prevalence of epileptic seizures in a large APP/PS1 mouse cohort. We have also systematically characterized different subtypes of epileptiforms spikes and their regional distribution in APP/PS1 mice.
Keywords
Leaders
Senior Researchers
Doctoral Researchers
-

Sara Häkli
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Elisa Hegnet
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Maria Gotkiewicz
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Kanishka Kanishka
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences


