
MINERVA
MINERVA is funded under the 2021 ERA-NET NEURON call for “Neurodevelopmental Disorders”. The project is supported through the following national funding organizations: Academy of Finland, Finland; Federal Ministry of Education and Research (BMBF), Germany; and Research Foundation – Flanders (FWO), Belgium. The Istituto Superiore di Sanità (ISS), Italy contributes to the project with own funds. The project will be carried out during 2022-2026.
Microglia/neuron crosstalk in autism spectrum disorder: Role of early inflammatory activation (MINERVA)
Autism spectrum disorder (ASD) is a complex condition of developmental origin that involves persistent challenges in social interaction, communication, restricted/repetitive behaviors and even cognition. The incidence of ASD has increased dramatically in recent years. This cannot be fully explained by changes in diagnostic criteria or methods suggesting that environmental factors contribute to the disease prevalence, possibly through stimulation of the immune system resulting in altered immune signatures and in particular, brain microglial functions.
While shaping the developing central nervous system (CNS) in temporal manner is the perfect task for the extremely versatile and mobile microglia, it leaves the developmental process vulnerable to external immunological insult. Indeed, (i) genetic and environmental immune-associated risk factors for ASD, (ii) immune alterations in individuals with ASD, and (iii) ASD symptom reduction in children with fever, point towards the involvement of the immune system in the pathophysiology of ASD which can disrupt microglial function and neural circuitry, and may contribute to altered social communication and other behavioral impairments in ASD.
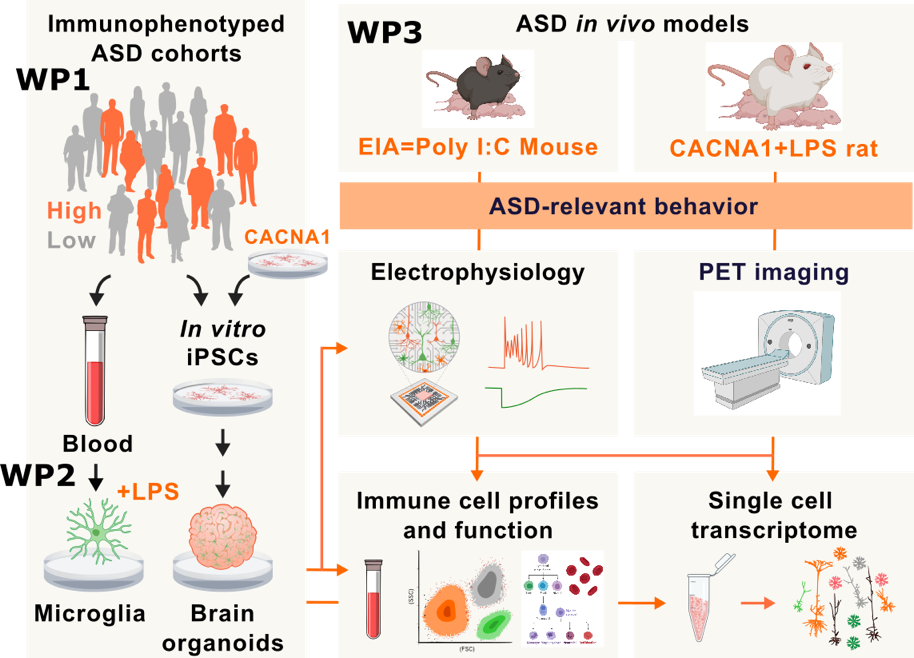
MINERVA brings together cross-national expertise from human-based modelling, state-of-the-art in vivo models with ASD-relevant behavioral measures, clinician scientists and commercialization of research, including patented therapies for CNS disorders to investigate how external immunological insults or genetic factors impair microglia-dependent neuronal development and functions. Unique, validated in vitro and in vivo models and deeply phenotyped individuals with ASD place us at the forefront for identification of neuroinflammatory mechanisms that shape ASD profile.
-
Immune activation during pregnancy is implicated in ASD pathogenesis. Maternal immune challenge induces sustained imprints in the immune system and microglial bioenergetic profile. These exert a profound influence on immune-metabolic function and behavior in offspring even in adult life. ASD is also strongly influenced by genetic factors that further drive microglial malfunctions, in particular CACNA1C mutations. The cross-disorder risk gene CACNA1C is implicated in the etiology of all ASD and single-nucleotide polymorphisms (SNPs) within CACNA1C are ranked among the best replicated and most robust genetic findings from genome-wide association studies in psychiatry. Gene-environment interaction has been proposed to be a determining element in the double hit-theory in ASD. However, it is unknown whether the alterations, induced by interaction of maternal infections and genetic risk factors cause persistent metabolic alterations, proinflammatory immune states, malfunction in microglia-neuron crosstalk and disturbed neural wiring in humans.
-
The primary objective of MINERVA is to investigate how early immune activation and its interaction with genetic factor (CACNA1C) drive microglia-neuron crosstalk during brain development and brain excitatory/inhibitory imbalance leading to lifelong consequences in modulating ASD phenotype and disease severity. We hypothesize that early immune activation and its interaction with genetic risk factors lead to behavioral abnormalities that are mediated by microglia malfunctions. We use double hit models consisting of maternal immune and postnatal immune activation and or the genetic risk of Cacna1c haploinsufficiency together with maternal immune activation to evaluate how altered immune signatures drive brain excitatory/inhibitory balance and development of ASD.
The secondary objectives of MINERVA are to:
- investigate how ASD alters immune cell heterogeneity and redox profiles and to compare peripheral immune profiles in humans with ASD to corresponding rodent models
- decipher how immune activation shapes microglia-neuron activation signatures and excitatory to inhibitory switch of GABA signaling in vivo and vitro
- elucidate whether interaction between genetic risk and early immune activation affects microglia-neuron activation signatures, neurodevelopment and brain excitatory/inhibitory imbalance
- test therapeutic interventions targeting ASD immuno-phenotypes with the goal to improve behavioral and cognitive impairments
- promote meaningful involvement of individuals with ASD
-
The project is divided into three related work packages (WPs). We utilize samples, cells and clinical data from individuals with ASD and state-of-the-art human-based in vitro and in vivo models to discover the molecular underpinnings of ASD and test potential treatment approaches. We carry out seminal studies to validate innovative in vitro and in vivo model systems to human ASD immune signatures for investigation of ASD-related neuroimmune activation, microglia-neuron interaction and brain excitatory/inhibitory imbalance. We aim for future immune stratification of individuals with ASD and for therapeutic strategies based on their immune signature.
-
ASD affects one in every 160 children worldwide. The burden of ASD to individuals and their relatives is lifelong, and thus ASD causes a significant socio-economic burden. Adults with ASD previously excluded from diagnosis of autism or misdiagnosed tend to live unrecognized in the society and are socioeconomically disadvantaged: they are poorly integrated into the mental health care system and there is limited evidence for remission in adulthood. A clinical product(s) would bring down socioeconomic cost of ASD care. The knowledge of novel mechanisms in ASD is also expected to have an impact on public policymaking and current guidelines and recommendations. MINERVA results will be also shared with individuals with ASD and their care givers. Understanding of ASD and underlying mechanisms gives “evidence-based” hope for ASD patients and their relatives and lessen the personal burden. At the same, MINERVA strives to reduce stigma and emotional impact around ASD by informing the general public and most relevant stakeholders about development in the field.
-
Tarja Malm, University of Eastern Finland, Finland (Project Coordinator)

Tarja Malm is Professor in Neuroinflammation at the University of Eastern Finland. The Malm group (https://sites.uef.fi/tarja-malm-group/) aims to understand inflammatory cell functions, especially those of microglia, and the mechanisms and mediators neuroinflammatory reactions. In this project our main focus is on functional fingerprints of microglia-neuron crosstalk in autism spectrum disorder. We use interdisciplinary approaches and novel, human based models to find novel therapeutic targets for the disease benefit and to discover novel biomarkers. Our lab develops novel human induced pluripotent stem cell-based models including microglia and organoids to investigate cellular response using omics approaches and electrophysiology. In addition, we combine animal modelling with functional outcomes to clinical patient material and data using bioinformatic approaches. The group consists of 7 postdoctoral researchers, 10 PhD students and a laboratory technician.
Judith Alferink, University of Münster and Clinic for Psychiatry and Psychotherapy, Alexianer GmbH, Münster, Germany

Judith Alferink is Professor for Immunobiology of Psychiatric Disorders at the Department of Mental Health of the University Hospital Münster (https://web.ukm.de/index.php?id=11807) and Head of the Clinic for Psychiatry and Psychotherapy, Alexianer Münster GmbH, Germany (https://www.alexianer-muenster.de/leistungen/kliniken/psychiatrie-und-psychotherapie-klinik-maria-brunn).
The main interest of her research group is to characterize the peripheral immune system and neuroinflammation in mental disorders. In clinical studies, the group defines peripheral immune signatures in major depression, autism, and anorexia nervosa related to severity and disease progression. The aim is to identify immune signatures in the peripheral blood that harbour predictive capacity for individual patients` disease course and treatment response. Underlying mechanisms and the impact of microglia, dendritic cells and their effector molecules on stress-induced and emotional behaviours are characterized in vivo using gene-defect and reporter mouse models. A second research topic of the laboratory is the characterization of immune alterations and neuroinflammation in Alzheimer’s disease. Here we use a mouse model of amyloidosis to characterize the influence of myeloid cells and chemokines on disease-associated immune processes. The group consists currently of one postdoctoral/senior position, two PhD students in experimental research, one technician, one psychologist, 5 MSc students of Psychology or (Bio-) Medicine.
Carsten Culmsee, University of Marburg, Germany

Carsten Culmsee is Professor for Clinical Pharmacy, Center for Mind, Brain and Behavior (CMBB), Institute for Pharmacology and Clinical Pharmacy, Philipps-University of Marburg (http://www.uni-marburg.de/fb16/ipkp/gruppen/ag_culmsee). Culmsee’s research interests focus on regulation of mitochondrial integrity and function in paradigms of cellular stress, neuro-inflammatory processes and metabolic impairment contributing to neuronal dysfunction in psychiatric disorders and neurodegenerative diseases. Major aims are to validate metabolic, neurobiological determinants and novel therapeutic targets in diseases of the nervous system. Projects in Clinical Pharmacy focus on drug safety issues and treatment optimization in age-related neurological diseases and neuropsychiatric diseases. In this project we use human cell culture, organoid systems and patient samples to evaluate metabolic regulation of impaired neuronal and neuro-inflammatory responses in model systems relevant to autism. Further, we evaluate metabolic and neuro-inflammatory determinants and potential pharmacological intervention in models of perinatal infection in CACNA1c rats. The group consists of two postdoctoral/senior positions, four PhD students in experimental research, five PhD students in clinical research, 1 Master student, 2 Bachelor students, two technicians and administrative staff.
Laura Ricceri, Istituto Superiore di Sanità, Rome, Italy

Laura Ricceri is Senior Researcher at Centre for Behavioural Sciences and Mental Health at Istituto Superiore di Sanità (https://www.iss.it/en/centro-di-riferimento-per-le-scienze-comportamentali-e-la-salute-mentale). Our group has a specific background in developmental psychobiology and is involved in research in the field of Behavioral Neuroscience, with a special interest in etiology of Neurodevelopmental disorders and early exposure to neurotoxicants. In this project, in tight collaboration with Marilena GRIGUOLI and Enrico CHERUBINI (European Brain Research Institute, Italy https://www.ebri.it/en/) we use models of double-hit perinatal infections for evaluation of i) microglia contribution to long-term behavioral and electrophysiological alterations, and ii) potential pharmacological intervention targeting cation-chloride co-transporters. The group consists of 1 postdoctoral researcher, 1 research fellow, 1 PhD student and 2 Master students.
Markus Wöhr, KU Leuven, Belgium

Markus Wöhr, Dr. rer. nat. (PhD), is Professor for Biological Psychology and Behavioral Pharmacology at KU Leuven, Belgium, and Young Investigator Group Leader at the Philipps-University of Marburg, Germany. He has a broad background in animal behavior and translational research models for neuropsychiatric dysfunctions, with specific training and expertise in behavioral neuroscience of affective and neurodevelopmental disorders. He is the head of the Social and Affective Neuroscience Research Group at the Faculty of Psychology and Educational Sciences, KU Leuven. The Wöhr Lab at KU Leuven (https://www.kuleuven.be/wieiswie/en/person/00140784) combines genetic, pharmacological, and behavioral approaches and studies neurobiological mechanisms underlying social behavior, acoustic communication through ultrasonic vocalizations, and socio-affective information processing in mice and rats. We focus on socio-affective dysfunctions in rodent models for affective and neurodevelopmental disorders, most notably autism. Our long-term goal is to improve translational research models through deciphering rodent socio-affective communication. The lab at KU Leuven currently consists of 2 postdoctoral researchers and 3 PhD students. For news, follow us on twitter: https://twitter.com/MarkusWohr
-
Annual Meeting 2025, June 16–17, 2025, Marburg, Germany
The third MINERVA Annual Meeting took place in sunny Marburg on June 16–17, 2025. Presentations by students, postdocs, and principal investigators highlighted the consortium’s productivity over the past year. They clearly demonstrated progress toward delivering a coherent and complementary set of research outcomes, as envisioned in the original project plan.
In addition to these internal presentations, Carsten Wotjak, a member of the external advisory board, delivered a talk. Valuable input was also provided by a representative from a patient organization, who offered important suggestions for future research directions.
As the project gradually approaches its conclusion, the most promising findings will be further studied with the support of additional funding.

Translational Research on Neurodevelopmental Disorders Workshop and MINERVA Annual Meeting 2024, May 27-28, 2024, Kuopio, Finland
The second MINERVA Annual Meeting took place in Kuopio on May 28, 2024. The meeting was joint with the workshop on neurodevelopmental disorders, which gathered a total of 60 participants. The busy program included presentations on gene-environment interactions in relation to autism by MINERVA partners, followed by perspectives from autistic individuals, educational professionals, and clinicians. At the end, we had the opportunity to hear updates from other Finnish ERA-NET NEURON projects led by Jaan-Olle Andressoo and Takashi Namba.

MINERVA partners and Heta Pukki, Autistic Spectrum Finland (left). Photo by Raija Törrönen.
Annual Meeting 2023, August 30 – September 1, 2023, Rome, Italy
The first MINERVA Annual Meeting was held in Rome on September 1, 2023. This was the first time we met face to face and the time together was immediately warm like a family meeting. We spent the day briefly summarizing the progress of planned experiments followed by a lively discussion. Thank you Laura and Enrico!

-
Coordinator: Professor Tarja Malm

Coordinator Affiliation and Contact Information:
A.I. Virtanen Institute for Molecular Sciences, University of Eastern Finland
Email: [email protected]

