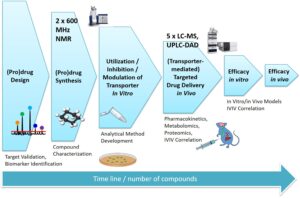
The designed prodrug molecules are synthesized, purified (recrystallization, chromatography), and characterized (NMR, MS, Elemental analysis) in our state-of-the-art synthesis laboratories.
Physicochemical and pharmaceutical properties (e.g. distribution coefficient (log D), aqueous solubility) and chemical stabilities at various pH values as well as plasma protein binding of the novel prodrugs are determined in our state-of-the-art analysis laboratories. The rate and quantity of the released parent drugs are evaluated in vitro in various enzyme-containing media (rodent and human-derived) and buffered solutions containing pure enzymes.
Transporter- mediated uptake rate and extend of (pro)drugs into the human cells are evaluated in suitable cell lines expressing the desired transporter. The analysis are carried out in updated analytical equipment (UPLC-DAD, HPLC-F or LC-MS/MS) after suitable sample preparation.
(Pro)drug pharmacokinetics and targeting efficacies are evaluated with more sophisticated in vivo studies in rodents. The analyses are carried as mentioned above and metabolic profiles of novel compounds are studied . Finally, in vitro-in vivo correlations of compound structure and targeting efficacy are projected.
Pharmacological efficacy (preventative and curative) and possible toxicological properties (biocompatibility, cytotoxicity, genotoxicity, etc.) of the developed (pro)drugs are studied firstly in vitro and secondly in vivo. Reliable biomarkers (already known but also possible novel biomarkers) are identified by LC-MS/MS (targeted/global proteomics/metabolomics). Finally, in vitro-in vivo correlations are projected and translated to human situation.