
Leinonen Retina Laboratory
Leaders
Hypothesis-driven, rigorously controlled and statistically powered visual neuroscience and pharmacology research.
Leinonen Retina Laboratory´s research focuses on two projects: (1) Homeostatic plasticity in the retina, its molecular mechanisms and implications for functional adaptation; (2) investigation of a novel drug repurposing-strategy for the treatment of retinal degenerations (RD). We use the retina as a model tissue for plasticity, pharmacology and drug discovery research for several reasons, such as amenability for noninvasive imaging due to eye’s transparency, finely layered structure and well-characterized structure-function relationships, functional similarity to that of brain due to same neurodevelopmental origin. Arguably, the retina is the best-characterized neuronal system in the body.
We detected retinal adaptability to sensory defect in year 2013 and our first paper related to this was published in 2020 (Leinonen et al., eLife). Next, we aim to solve the molecular pathways enabling retinal homeostatic plasticity. This knowledge will help to find mechanisms how the CNS adapts to injury and malfunction and can guide pharmacotherapies.
Our drug discovery program uses repurposed GPCR drugs. We have shown that a treatment with a combination therapy comprised of three GPCR-drugs slows progressive RD. The next crucial task is to uncover the therapeutic mechanisms of our therapy. This will facilitate further development of the treatment concept and can launch innovative drug development pipelines.
Cooperation
-
Distinguished University Professor, Department of Chemistry, University of Pittsburgh, USA
-
Distinguished Professor, Chair of Ophthalmology, University of California Irvine, USA
-
Assistant Professor of Ophthalmology, Harvard University, Boston Children´s Hospital, USA
-
Professor, College of Veterinary Medicine, Michigan State University, USA
-
Assistant Professor, Ophthalmology & Visual Sciences, University of Utah, USA
-
Principal Investigator, Polish Academy of Science, International Centre for Experimental Eye Research, Poland
-
Laboratory Chief, National Cerebral and Cardiovascular Center, Osaka, Japan
-
Professor, Dean, Faculty of Biochemistry and Molecular Medicine, University of Oulu, Finland
-
Assistant Professor, Physiology and Biophysics, University of California Irvine, USA
-
Assistant Professor, Ophthalmology, University of California Irvine, USA
-
Professor, Ophthalmology, University of California Irvine, USA
-
 Neuropharmacology and Drug Targets Consortium (NDTC) 01.01.2022 -
Neuropharmacology and Drug Targets Consortium (NDTC) 01.01.2022 - -
 Ocular Drug Delivery group (ODD) 01.01.2010 -
Ocular Drug Delivery group (ODD) 01.01.2010 - -
 Kaarniranta AMD Lab 01.01.2004 -
Kaarniranta AMD Lab 01.01.2004 - -
 Neuroinflammation research group 01.01.2017 -
Neuroinflammation research group 01.01.2017 - -
 Neurobiology of Memory 12.11.2022 -
Neurobiology of Memory 12.11.2022 - -
 Biomedical Image Analysis 01.11.2016 -
Biomedical Image Analysis 01.11.2016 - -
 Transporter-Targeted Drug Delivery 01.09.2012 -
Transporter-Targeted Drug Delivery 01.09.2012 -
RESEARCH DESCRIPTION
The laboratory leader´s interest towards the eye´s retina was initially based on the realization that the retina manifests pathological changes in diseases that are primary known as brain diseases (such as Alzheimer´s and Parkinson´s Diseases). The goal of this research branch is to develop retina-based biomarkers for brain diseases. Hopefully, with the help of retina-based biomarkers, these diseases could be diagnosed in an early disease phase, or even before clinical disease manifestation.
The laboratory´s research project (1) follows up my PhD project’s idea that the retina of the eye provides a window to study central nervous system, here in particular from neuroplasticity point of view. Our recently published data (Leinonen et al. 2020, eLife) show that according to mass retinal electrophysiology responses, signal transmission from photoreceptors (PR) to rod bipolar cells (BC) is potentiated upon PR decline. This is seen in mice with PR degenerative disease and by pharmacological suppression of PR function in healthy normal mice. The potentiation in PR-BC transmission is associated with reorganization of synaptic proteins as shown by expression analyses. Furthermore, despite dying PRs, behavioral visual function is well-maintained in a mouse model of retinitis pigmentosa. We believe that the inner retina responds to sensory deprivation by means of homeostatic plasticity, which helps to maintain function at brain level. Our current focus is to decipher the molecular mechanisms of retinal homeostatic plasticity.
The objective of the research project (2) is to test pharmacological treatments based on a systems pharmacology approach in rescuing retinal photoreceptors in various mouse models of retinal degenerative diseases (including Leber congenital amaurosis, Retinitis Pigmentosa and dry AMD). Inherited and idiopathic retinal degenerative diseases, including the dry form of age-related macular degeneration (AMD) and Retinitis Pigmentosa (RP), generally lack treatments that would halt the progression of vision loss after diagnosis. In most retinal degenerative diseases, the retinal pigment epithelium and rod photoreceptors degenerate first followed by damage to cone photoreceptors and remodeling of the inner retina. As for human vision, the preservation of cone photoreceptors and inner retina would suffice to retain visual acuity even if the rods were partially lost.
Our systems pharmacology approach utilizes a combined therapy comprised of existing clinical drugs that act on G protein-coupled receptors (GPCRs). We have shown that prophylactic treatment with a targeted combination therapy with several simultaneously administered GPCR-acting drugs at low doses (sub-therapeutic if given as monotherapy) can prevent acutely induced retinal degeneration in distinct mouse models of retinal degenerative diseases. We are pursuing this promising drug development pipeline by expanding the trials into more translationally relevant progressive retinal degenerative disease models that differ in etiology, but which have the same blinding endpoint, and finally to initial clinical trials. A subsequent crucial task is to uncover the exact molecular mechanisms that can explain the retinal protective effects of our combination therapy. This will facilitate in further development of the treatment concept, and can launch innovative novel drug development pipelines.
Drug repurposing approach re-evaluates the usefulness of drugs that have already survived the costly drug development pipeline, and have proven to be safe for the use in humans. Drug repurposing has the potential to provide an economical and an effective alternative to treat patients suffering from disparate retinal degenerative diseases, and may be useful in the treatment of other complex neuronal diseases as well. Most importantly, the use of approved drugs surpasses several formally required drug development phases, and can therefore provide novel clinical remedies fast.
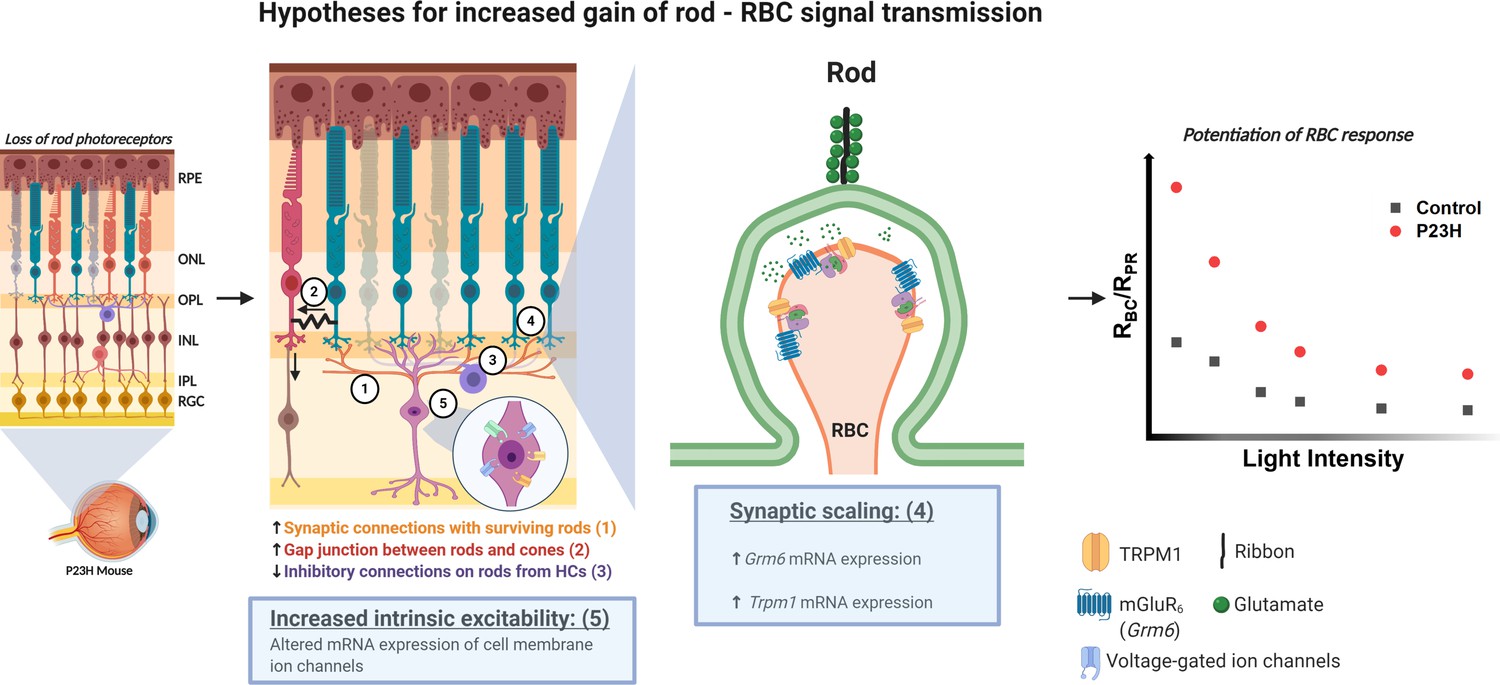
Homeostatic plasticity in the retina - mechanistic hypothesis
Keywords
Leaders
Post-doctoral Researchers
Doctoral Researchers
-

Umair Seemab
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences -

Katri Vainionpää
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences -

Anthi-Styliani Makiou
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences -

Anna Kalatanova
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences
Technicians
Publications
6 items-
Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing
Suh, Susie; Choi, Elliot H; Leinonen, Henri; Foik, Andrzej T; Newby, Gregory A; Yeh, Wei-His; Dong, Zhiqian; Kiser, Philip D; Lyon, David C; Liu, David R; Palczewski, Krzysztof. 2021. Nature biomedical engineering. 5: 169-178 -
Homeostatic plasticity in the retina is associated with maintenance of night vision during retinal degenerative disease
Leinonen, Henri; Pham, Nguyen C; Boyd, Taylor; Santoso, Johanes; Palczewski, Krzysztof; Vinberg, Frans. 2020. eLife. 9: e59422 -
A Mixture of U.S. Food and Drug Administration-Approved Monoaminergic Drugs Protects the Retina From Light Damage in Diverse Models of Night Blindness
Leinonen H, Choi EH, Gardella A, Kefalov VJ, Palczewski K.. 2019. Investigative ophthalmology and visual science. 60: 1442-1453 -
A Combination of G Protein-Coupled Receptor Modulators Protects Photoreceptors from Degeneration
Orban T, Leinonen H, Getter T, Dong Z, Sun W, Gao S, Veenstra A, Heidari-Torkabadi H, Kern TS, Kiser PD, Palczewski K.. 2018. Journal of pharmacology and experimental therapeutics. 364: 207-220 -
Vision in laboratory rodents - Tools to measure it and implications for behavioral research
Leinonen, H; Tanila, H. 2018. Behavioural brain research. 352: 172-182 -
Electrophysiology of visual pathways as a screening tool for neurodegenerative diseases: evidence from mouse disease models
Leinonen Henri. 2016. Publications of the University of Eastern Finland. Dissertations in Health Sciences



