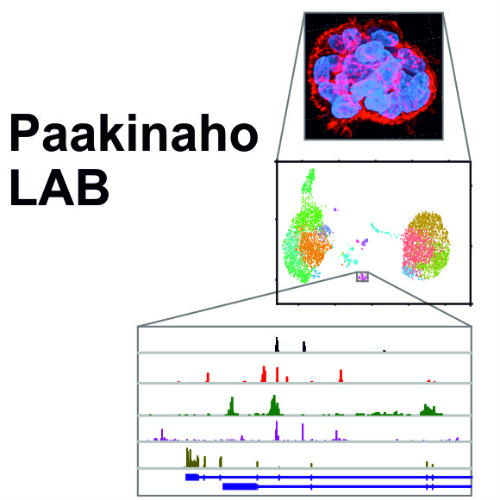
Transcription factor crosstalk in cancers - Paakinaho lab
Leaders
Our research aims to decipher the key transcriptional mechanism mediated by steroid receptors and pioneer factors in health and disease. Our specific interests are with transcription factor crosstalk in prostate cancer; how steroid receptors and pioneer factors alter each other’s activity impacting cancer initiation, development, and drug resistance. Special emphasis is placed from the steroid receptors on the glucocorticoid receptor (GR) and androgen receptor (AR), and from the pioneer factors on the forkhead box A1 (FOXA1) and activator protein 1 (AP-1). In our research we utilize genome-wide deep sequencing, chromatin proteomic, single-cell genomic, and CRISPR genome editing techniques. We aim to discover new therapeutic targets and forms of treatment to overcome advanced and drug therapy resistant cancers. Our findings may also be applicable to research in other disease areas, including metabolic disorders.
Research group:
Ville Paakinaho
Academy Research Fellow, Assistant Professor
PhD, Title of a Docent (molecular endocrinology)
Doctoral researcher, MSc Laura Helminen
Doctoral researcher, MSc Johannes Hiltunen
Doctoral researcher, MSc Jasmin Huttunen
Project researcher, MSc Heini Sohlberg
Research projects:
(i) Transcription factor crosstalk mechanism in benign versus malignant prostate cancer cells.
(ii) Steroid receptor-mediated drug therapy resistance in prostate cancer cells.
(iii) Coregulators in transcription factor crosstalk and their impact in therapy naïve and drug therapy resistant prostate cancer cells.
Research results:
Our recent research has demonstrated that FOXA1 plays a crucial role in GR-mediated drug resistance and that transcription factor crosstalk can be leveraged to counteract its harmful effects in prostate cancer (Helminen et al., 2024, Nucleic Acids Res; Huttunen et al., 2024, Cell Mol Life Sci). We have also discovered that GR restricts the progress of prostate cancer in the presence of AR, while it mediates the survival of the cancer in the absence of AR (Hiltunen et al., 2025, Genome Res).
Popularized news from our research results:
9th of June 2025: The glucocorticoid receptor is a double-edged sword in prostate cancer
9th of April 2024: New insight into combatting drug-resistant prostate cancer
News
-
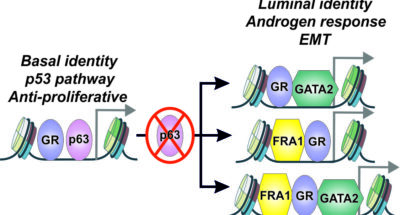 Preprint publication: bioRxiv
Preprint publication: bioRxivPreprint publication: bioRxiv
Molecular switch mediates the glucocorticoid receptor transition from tumor suppressor to oncogene in the prostate -
 The glucocorticoid receptor is a double-edged sword in prostate cancer
The glucocorticoid receptor is a double-edged sword in prostate cancerThe glucocorticoid receptor is a double-edged sword in prostate cancer
A recent study from the Institute of Biomedicine at the University of Eastern Finland shows that the glucocorticoid receptor can both promote and… -
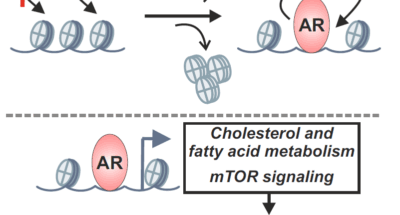 Research publication: Genome Res
Research publication: Genome ResResearch publication: Genome Res
Androgen receptor-mediated assisted loading of the glucocorticoid receptor modulates transcriptional responses in prostate cancer cells
Our research goals and results
For background information, see the following review articles related to our research topics:
2018 Swinstead, Paakinaho, Hager: Chromatin reprogramming in breast cancer
Research goals
Our research aims to decipher several aspects in transcription factor (TF) crosstalk:
(i) What kind of crosstalk mechanism occur between steroid receptors and pioneer factors in benign versus malignant prostate cancer cells.
(ii) How and through what mechanism does GR replace AR signaling in antiandrogen-resistant prostate cancer cells.
(iii) To what degree does coregulators influence TF crosstalk in therapy naïve and antiandrogen-resistant prostate cancer cells.
(iv) Which prostate cancer TF crosstalk mechanisms can be observed in other cancer cell types.
We anticipate that our findings will open new therapeutic avenues for targeting coordinated TF activity in the prostate. Specifically, dual targeting of steroid receptors, pioneer factors, and their coregulators may offer a strategy to overcome drug resistance commonly observed with single-agent therapies. Additionally, modulating GR activity through alternative signaling pathways in antiandrogen-resistant prostate cancer could preserve the systemic benefits of glucocorticoids while minimizing their oncogenic potential. Importantly, we expect that the mechanistic insights gained from this study can be extended to other cancer types, and potentially to non-cancerous diseases, such as metabolic disorders, where TFs play critical regulatory roles.
Research results
For Research aim (i), we have published our results in Genome Res -journal:
The datasets associated with the publication can be accessed here: GSE266217
For Research aim (ii), we have published our results in Nucleic Acids Res -journal:
The datasets associated with the publication can be accessed here: GSE214757
For Research aim (iii), we have published our results in Cell Mol Life Sci -journal:
The datasets associated with the publication can be accessed here: GSE245969
Lab news
2026-02: Laura received doctoral research grant from Instrumentarium Science Foundation and the Finnish Cultural Foundation.
2025-11: Ville presented the results from the research group at 34th Annual ARTP meeting.
2025-10:

2025-09: Jasmin and Johannes presented their results at iCAN Symposium.
2025-08: The second bioRxiv preprint for Research project (i) is published.
2025-06: Research project (i) results are published in Genome Res -journal. Popularized news related to our research is publieshed at uef.fi website.
2024-11: bioRxiv preprint for Research project (i) is published. Jasmin was selected to 3-year Doctoral education pilot in precision cancer medicine (iCANDOC) program.
2024-09: Johannes and Laura presented their results at EMBO Nuclear Receptor meeting.
2024-07: Review article from our research field is published in Front Endocrinol -journal.
2024-04: Research project (iii) results are published in Cell Mol Life Sci -journal. Popularized news related to our research is publieshed at uef.fi website.
2023-11: Research project (ii) results are published in Nucleic Acids Res -journal. Laura presented her research results at EMBL Cancer genomics meeting.
2023-10

2023-08: Johannes presented his research results at FEBS Epigenomics, Nuclear Receptors and Diseases meeting.
2023-06: Ville presented the results from the research group at ENDO 2023 meeting.
2023-03: bioRxiv preprint for Research project (ii) is published.
-
- Research Council of Finland (decision) (2021-2026)
- Sigrid Jusélius Foundation (2019-2021, 2022-2023, 2024-2026)
- Cancer Foundation Finland (2020, 2022-2023, 2025)
- UEF strategic funding (2022-2024)
Keywords
Leaders
Doctoral Researchers
-

Laura Helminen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences -
Johannes Hiltunen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences -
Jasmin Huttunen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences
Supporting Staff
Publications
4 items-
Androgen receptor-mediated assisted loading of the glucocorticoid receptor modulates transcriptional responses in prostate cancer cells
Hiltunen, Johannes; Helminen, Laura; Aaltonen, Niina; Launonen, Kaisa-Mari; Laakso, Hanna; Malinen, Marjo; Niskanen, Einari A; Palvimo, Jorma J; Paakinaho, Ville, 2025, Genome research, 35, 8, 1717-1732. A1 Journal article (refereed), original research -
EP300/CREBBP acetyltransferase inhibition limits steroid receptor and FOXA1 signaling in prostate cancer cells
Huttunen, Jasmin; Aaltonen, Niina; Helminen, Laura; Rilla, Kirsi; Paakinaho, Ville, 2024, Cellular and molecular life sciences, 81, 1, 160. A1 Journal article (refereed), original research -
Glucocorticoid receptor action in prostate cancer: the role of transcription factor crosstalk
Hiltunen, Johannes; Helminen, Laura; Paakinaho, Ville, 2024, Frontiers in endocrinology, 15, 1437179. A2 Review article, Literature review, Systematic review -
Chromatin accessibility and pioneer factor FOXA1 restrict glucocorticoid receptor action in prostate cancer
Helminen, Laura; Huttunen, Jasmin; Tulonen, Melina; Aaltonen, Niina; Niskanen, Einari A; Palvimo, Jorma J; Paakinaho, Ville, 2023, Nucleic acids research, 52, 2, 625-642. A1 Journal article (refereed), original research

