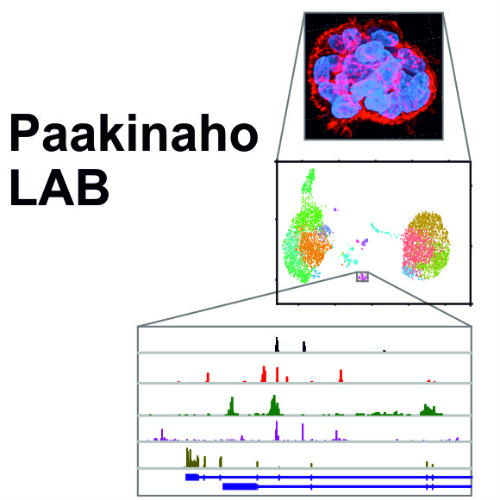
Transcription factor crosstalk in cancers - Paakinaho lab
Leaders
Our research aims to decipher the key transcriptional mechanism mediated by steroid receptors and pioneer factors in health and disease. Our specific interests are with transcription factor crosstalk in prostate cancer; how steroid receptors and pioneer factors alter each other’s activity impacting cancer initiation, development, and drug resistance. Special emphasis is placed from the steroid receptors on the glucocorticoid receptor (GR) and androgen receptor (AR), and from the pioneer factors on the forkhead box A1 (FOXA1) and activator protein 1 (AP-1). In our research we utilize genome-wide deep sequencing, chromatin proteomic, single-cell genomic, and CRISPR genome editing techniques. We aim to discover new therapeutic targets and forms of treatment to overcome advanced and drug therapy resistant cancers.
Research group:
Ville Paakinaho
Academy Research Fellow, PhD, Title of a Docent (molecular endocrinology)
Project researcher, PhD Niina Aaltonen
Doctoral researcher, MSc Laura Helminen
Doctoral researcher, MSc Johannes Hiltunen
Doctoral researcher, MSc Jasmin Huttunen
Research trainee, MSc Mitali Mishra
Research trainee, BSc Heini Sohlberg
Research projects:
(i) Transcription factor crosstalk mechanism in benign versus malignant prostate cancer cells.
(ii) Steroid receptor-mediated drug therapy resistance in prostate cancer cells.
(iii) Coregulators in transcription factor crosstalk and their impact in therapy naïve and drug therapy resistant prostate cancer cells.
Research results:
Our recent research has demonstrated that FOXA1 plays a crucial role in GR-mediated drug resistance and that transcription factor crosstalk can be leveraged to counteract its harmful effects in prostate cancer (Helminen et al., 2024, Nucleic Acids Res; Huttunen et al., 2024, Cell Mol Life Sci). We have also discovered that GR restricts the progress of prostate cancer in the presence of AR, while it mediates the survival of the cancer in the absence of AR (Hiltunen et al., 2025, Genome Res).
News
-
 The glucocorticoid receptor is a double-edged sword in prostate cancer
The glucocorticoid receptor is a double-edged sword in prostate cancerThe glucocorticoid receptor is a double-edged sword in prostate cancer
A recent study from the Institute of Biomedicine at the University of Eastern Finland shows that the glucocorticoid receptor can both promote and… -
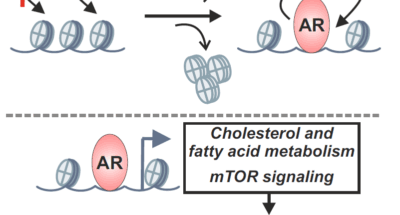 Research publication: Genome Res
Research publication: Genome ResResearch publication: Genome Res
Androgen receptor-mediated assisted loading of the glucocorticoid receptor modulates transcriptional responses in prostate cancer cells -
 New insight into combatting drug-resistant prostate cancer
New insight into combatting drug-resistant prostate cancerNew insight into combatting drug-resistant prostate cancer
New research from the University of Eastern Finland sheds light on the significance of the glucocorticoid receptor in drug-resistant prostate cancer,…
Detailed description of our research
Background
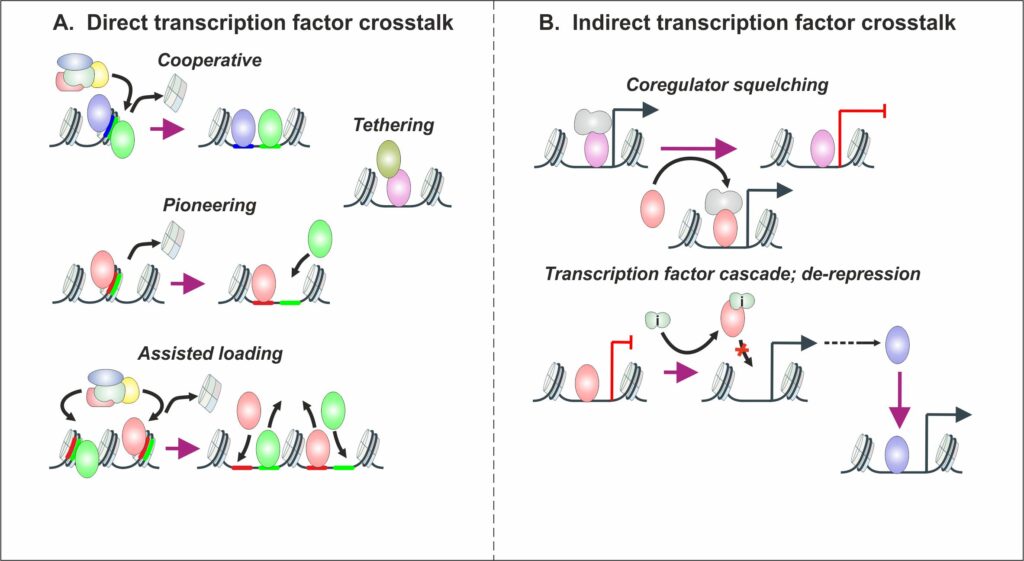
Transcriptional regulation is one of the most important biological processes in organisms contributing to every aspect of health and disease. Abnormal regulation of transcription can lead to several different disease states, with the most drastic alterations occurring in cancers. Regulation of gene expression through enhancers and promoters is mediated by transcription factors (TFs). At these sites, TFs influence transcriptional regulation by altering the chromatin accessibility and RNA polymerase action via changing the activity and recruitment of other TFs and coregulators. In the past, the action of many TFs has only been investigated in isolation of other TFs/signaling pathways. However, TF practically never work in isolation, wherein only one TF is acting while others remain inactive. Recently it has been indicated that the interplay of TFs can drastically change when multiple pathways are activated in sequence or simultaneously. There are multiple mechanisms how TFs can modulate chromatin binding and the activity of other TFs, such as cooperative binding, tethering, and assisted loading (Figure 1A, see below). In addition, there are indirect mechanisms, such as coregulator squelching and TF cascades (Figure 1B, see below). Interestingly, this TF crosstalk can influence both the progression and drug resistance of cancers as well as the regulation of normal metabolic processes.
Steroid receptors, such as estrogen (ER), androgen (AR), and glucocorticoid receptor (GR), are hormone-controlled TFs that regulate transcription in multiple central cellular systems. The action of steroid receptors have a major role in the development and progression diseases, especially hormone-dependent cancers. The AR influences the progression of prostate cancer (PCa), while the ER (ESR1) is a critical factor for breast cancer (BCa) development. Thus, both receptors can be considered as oncogenes, and are the major drug targets in these cancers; the ER and the AR are targeted by antagonist compounds, or the synthesis of their activating hormones is inhibited. E.g., in PCa, the AR action can be inhibited by enzalutamide (ENZ), a second-generation antiandrogen that competes with androgens for binding to the receptor’s ligand-binding domain. However, despite the effectiveness of these therapies, the cancers frequently bypass the blockade of ER and AR activity through development of drug resistance.
In contrast to ER and AR, GR has long been thought to have a tumor suppressor role, since the receptor can suppress the growth of cancer cells, as exemplified by the effect of glucocorticoids on lymphoblastic leukemia. Furthermore, glucocorticoids are widely used to alleviate therapy-related side-effects and to reduce inflammation in BCa and PCa patients. Despite the beneficial effects of glucocorticoids, recent evidence suggests that GR plays a detrimental role in both BCa and PCa. In BCa, the activation of GR enhances the metastasizing capability of the disease. In PCa, GR is postulated to be an essential player in therapy naïve and antiandrogen-treated cancer. Thus, GR possess both oncogenic and tumor suppressive activities.
Coregulators recruited by the ER and AR have been considered as potential targets for cancer therapies. The druggable coregulators include EP300 and various proteins of the bromodomain and extra-terminal (BET) family. Inhibition of the activity of these coregulators have been shown to restrict the action of AR in PCa. In addition to coregulators, pioneer factors, a class of TFs, can have a major impact on the activity of AR and ER in in PCa and BCa, respectively. Especially, the forkhead box A1 (FOXA1), which is frequently mutated and dysregulated in these cancers, is important regulator of steroid receptor chromatin binding. Interestingly, this regulatory capability occurs in symmetric enhancer-specific fashion wherein steroid receptors can also modulate the chromatin occupancy of FOXA1. Finally, also other steroid receptors, have emerged as important “coregulators” for the ER and AR in cancer cells. In PCa, the inhibition of AR by ENZ increases the expression of GR, which can lead to GR replacing AR as the driver of PCa progression. Thus, the TF crosstalk can have a major impact on the development and progression of not only therapy naïve but also antiandrogen-resistant PCa.
For more information, see our two review articles on these aspects:
Research goals
Our research aims to decipher several aspects in TF crosstalk (Figure 2):
(i) What kind of crosstalk mechanism occur between steroid receptors and pioneer factors in benign versus malignant PCa cells.
(ii) How and through what mechanism does GR replace AR signaling in antiandrogen-resistant PCa cells.
(iii) To what degree does coregulators influence TF crosstalk in therapy naïve and antiandrogen-resistant PCa cells.
(iv) Which PCa TF crosstalk mechanisms can be observed in other cancer cell types.
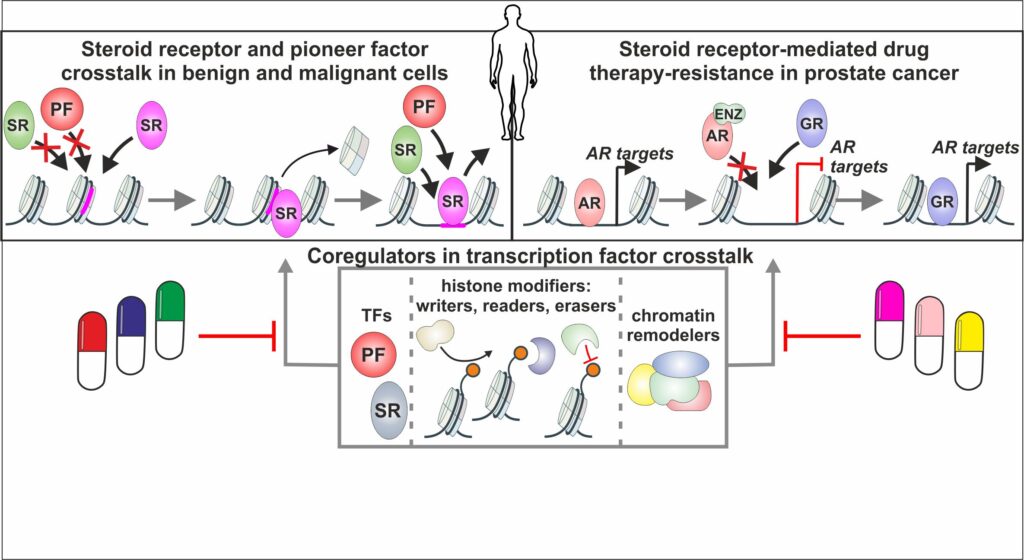
We expect to discover new therapeutic avenues to target concerted TF action in PCa. The concomitant targeting of steroid receptor with a coregulator could help to resolve drug resistance occurring with single drug treatments. In addition, targeting of GR in antiandrogen-resistant PCa through other signaling pathways could help retain the beneficial systemic effect of glucocorticoids. Finally, we anticipate that our discoveries in PCa can be expanded and utilized in other cancers.
Research results
For Research aim (i), we have published our results in Genome Res -journal:
The datasets associated with the publication can be accessed here: GSE266217
For Research aim (ii), we have published our results in Nucleic Acids Res -journal:
The datasets associated with the publication can be accessed here: GSE214757
For Research aim (iii), we have published our results in Cell Mol Life Sci -journal:
The datasets associated with the publication can be accessed here: GSE245969
Lab news
2025-06
Research project (i) results are published in Genome Res -journal.
2024-11
bioRxiv preprint for Research project (i) is published.
2024-09
Johannes and Laura presented their results at EMBO Nuclear Receptor meeting.
2024-07
Review article from our research field is published in Front Endocrinol -journal.
2024-04
Research project (iii) results are published in Cell Mol Life Sci -journal.
2023-11
Research project (ii) results are published in Nucleic Acids Res -journal.
Laura presented her research results at EMBL Cancer genomics meeting.
2023-10

2023-08
Johannes presented his research results at FEBS Epigenomics, Nuclear Receptors and Diseases meeting.
2023-06
Ville presented the results from the research group at ENDO 2023 meeting.
2023-03
bioRxiv preprint for Research project (ii) is published.
-
- Research Council of Finland (decision)
- Sigrid Jusélius Foundation
- Cancer Foundation Finland
- UEF strategic funding
Keywords
Leaders
Post-doctoral Researchers
Doctoral Researchers
-

Laura Helminen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences -
Johannes Hiltunen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences -
Jasmin Huttunen
Doctoral ResearcherInstitute of Biomedicine, School of Medicine, Faculty of Health Sciences
Supporting Staff
Publications
3 items-
EP300/CREBBP acetyltransferase inhibition limits steroid receptor and FOXA1 signaling in prostate cancer cells
Huttunen, Jasmin; Aaltonen, Niina; Helminen, Laura; Rilla, Kirsi; Paakinaho, Ville. 2024. Cellular and molecular life sciences. 81: . 160 A1 Journal article (refereed), original research -
Glucocorticoid receptor action in prostate cancer: the role of transcription factor crosstalk
Hiltunen, Johannes; Helminen, Laura; Paakinaho, Ville. 2024. Frontiers in endocrinology. 15: . 1437179 A2 Review article, Literature review, Systematic review -
Chromatin accessibility and pioneer factor FOXA1 restrict glucocorticoid receptor action in prostate cancer
Helminen, Laura; Huttunen, Jasmin; Tulonen, Melina; Aaltonen, Niina; Niskanen, Einari A; Palvimo, Jorma J; Paakinaho, Ville. 2023. Nucleic acids research. 2024; 52: 625-642 A1 Journal article (refereed), original research

