Leaders
Group description
The Molecular Medicine research group, led by Professor Seppo Ylä-Herttuala, is focused on developing new gene and protein-based approaches for the treatment of cardiovascular diseases, malignant glioma and diseases associated with disturbances in vascular biology. Our group was the first in the world to use direct adenoviral gene transfer to human arteries in vivo in 1996. Major achievements include the discovery of the vasculoprotective effect of VEGF gene therapy, characterization of the vascular effects of the new members of the VEGF family including their effects on lymphatic vessels, and identification of several new candidate genes for the treatment of vascular diseases.
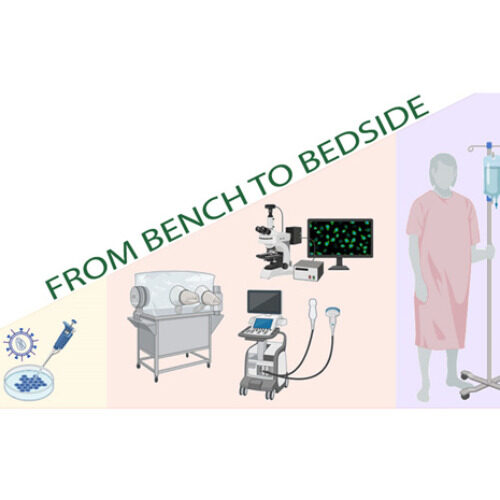
“Our greatest strength is from bench to bedside approach to research. We have the knowledge and facilities for developing new, innovative therapeutic solutions that can be first tested in small and large animal models before moving to clinical trials. Having the National Virus Vector Laboratory, in vitro and in vivo research facilities and Kuopio University Hospital within the same campus area, gives us an excellent platform for translational research,” Professor Seppo Ylä-Herttuala says.
So far, we have conducted eight phase I/II/III clinical studies with vectors developed in our group. Within our facilities in A.I. Virtanen Institute, we have the National Virus Vector Laboratory for viral productions. With the expertise gained over these years and the excellent infrastructure that we have, we were also able to promptly respond to the need for a COVID-19 vaccine during the ongoing pandemic and are in the process of testing one in a collaborative effort.
The scope and expertise of our group ensures a supportive and collaborative research environment in which we all strive for innovative and translational solutions for important global diseases. Our projects are varied and traverse from viral vectors to clinical trials so there is something for everyone. This also means that there is a lot of expertise and different skills within the group. What a great learning opportunity for motivated students and researchers!
UEF collaborating groups
Cooperation
-
 Cardiovascular Genomics (Kaikkonen lab) 01.09.2015 -
Cardiovascular Genomics (Kaikkonen lab) 01.09.2015 - -
 Molecular Modeling and Drug Design Research Group 01.01.2010 -
Molecular Modeling and Drug Design Research Group 01.01.2010 - -
 Ocular Drug Delivery group (ODD) 01.01.2010 -
Ocular Drug Delivery group (ODD) 01.01.2010 - -
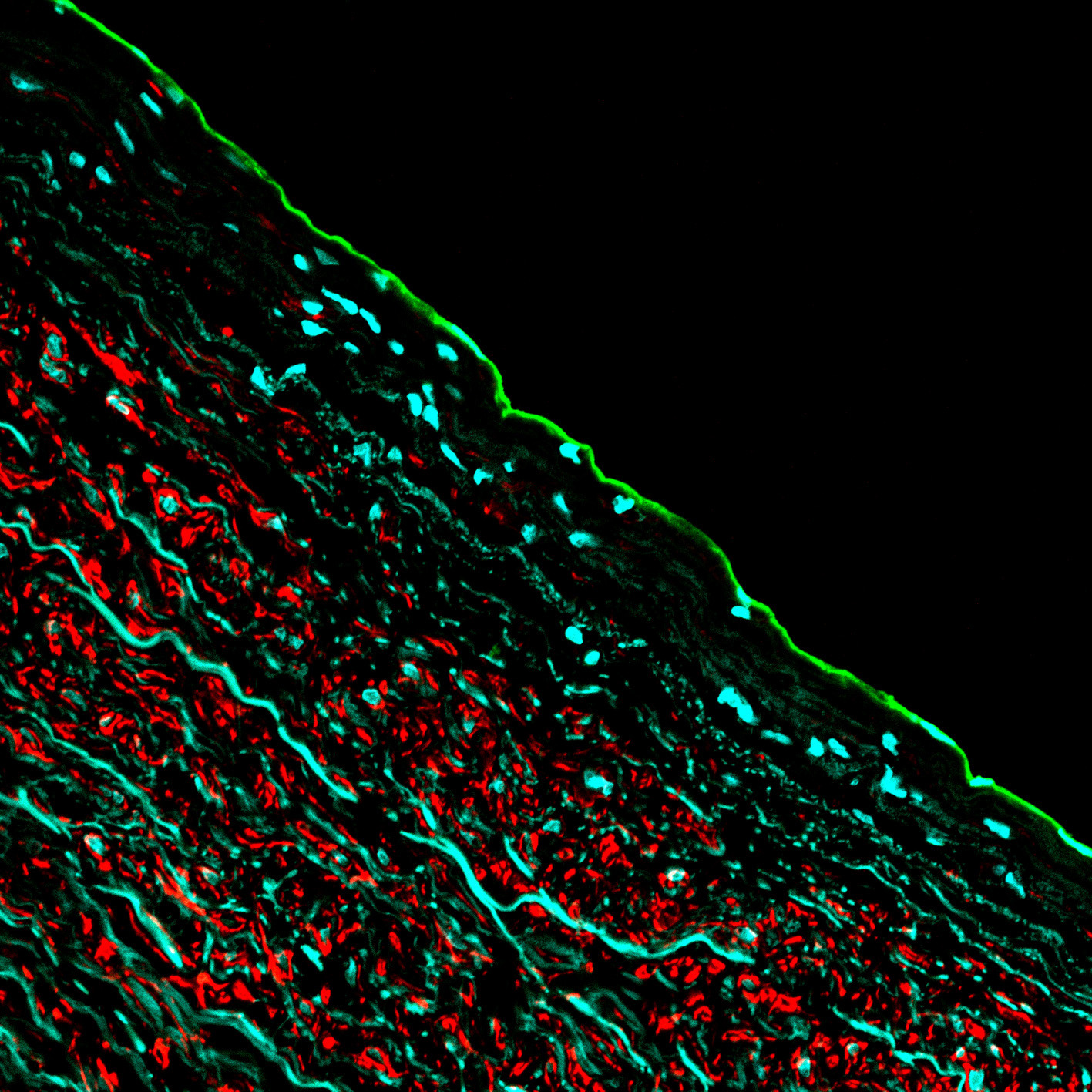 Vascular Biology (Laakkonen) 01.09.2019 -
Vascular Biology (Laakkonen) 01.09.2019 -
Projects
-
 Our focus is mainly translational – we are a gateway on the road from bench to bedside. Our team develops clinically relevant large in vivo disease models (pig) for myocardial ischemia and heart failure that we utilize in our research for novel therapies. We also use novel imaging modalities such as angiographic imaging and PET in our studies, some in collaboration with Kuopio University Hospital. In addition, we investigate delivery methods, effectiveness and feasibility of our myocardial gene therapy vectors that include angiogenic growth factors in large in vivo models.
Our focus is mainly translational – we are a gateway on the road from bench to bedside. Our team develops clinically relevant large in vivo disease models (pig) for myocardial ischemia and heart failure that we utilize in our research for novel therapies. We also use novel imaging modalities such as angiographic imaging and PET in our studies, some in collaboration with Kuopio University Hospital. In addition, we investigate delivery methods, effectiveness and feasibility of our myocardial gene therapy vectors that include angiogenic growth factors in large in vivo models.People involved
- Jussi Nurro, Juho Pajula, Henna Korpela, Jaakko Lampela, Jonna Räisänen, Kaisa Valli, William Welsh, Niko Järveläinen, Satu Siimes, Minja Heikkilä, Anni Määttä, Hanna Wang, Petri Mäkinen, Seppo Ylä-Herttuala
Collaborators
- Kuopio University Hospital, Turku PET Center
Selected publications
- Pajula, J. J., Halonen, P. J., Hätinen, O. P., Ylä-Herttuala, S., & Nurro, J. (2020). Adenoviral Gene Transfer of Gremlin Modulates Vascular Endothelial Growth Factor-A-Induced Angiogenesis in Porcine Myocardium. Human Gene Therapy, 31 (3–4), 211–218.
- Lähteenvuo, J., Hätinen, O. P., Kuivanen, A., Huusko, J., Paananen, J., Lähteenvuo, M., … Ylä-Herttuala, S. (2020). Susceptibility to Cardiac Arrhythmias and Sympathetic Nerve Growth in VEGF-B Overexpressing Myocardium. Molecular Therapy, 28 (7), 1731–1740.
- Hätinen, O. P. A., Lähteenvuo, J. E., Korpela, H. J., Pajula, J. J., & Ylä-Herttuala, S. (2019). Isolation of fresh endothelial cells from porcine heart for cardiovascular studies: A new fast protocol suitable for genomic, transcriptomic and cell biology studies. BMC Molecular and Cell Biology 20 (1).
-
 In the Academy of Finland funded COVID-19 vaccine project, we aim to develop an adenoviral vector vaccine against SARS-CoV-2. Our adenovirus vector expresses part of the SARS-CoV-2 spike protein. Once the vaccine is administered as a nasal spray it is estimated to produce strong immunity against the disease.
In the Academy of Finland funded COVID-19 vaccine project, we aim to develop an adenoviral vector vaccine against SARS-CoV-2. Our adenovirus vector expresses part of the SARS-CoV-2 spike protein. Once the vaccine is administered as a nasal spray it is estimated to produce strong immunity against the disease.People involved
- Emma Koivulehto, Ahmed Tawfek, Aino Sormunen, Erika Gurzeler-Tiihonen, Yegor Korzyukov, Nihay Laham-Karam, Tuija Kekarainen, Petri Mäkinen, Seppo Ylä-Herttuala
Collaborators
-
We are investigating malignant brain tumors. In these projects we focus on glioblastoma. We are determing its characteristics and aiming for the discovery of new cancer biomarkers. In addition, we are using multiple approaches to modeling disease and importantly we utilize patient material. More detailed information of biomarkers may benefit patient diagnostics and help to select better and personalized therapies in the future.
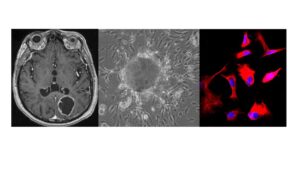
Magnetic resonance imaging of glioblastoma patient before surgery (modified from Niittykoski et al. 2017), patient derived glioma cells in culture and stained patient derived glioma cells. People involved
Collaborators
- Vincenzo Cerullo (University of Helsinki), Dario Greco (Tampere University Foundation Sr), Tuija Kekarainen (KCT), Olli-Pekka Kämäräinen (Kuopio University Hospital)
Selected publications
- Niittykoski M,von und zu Fraunberg M, Martikainen M, Rauramaa T, Immonen A, Koponen S, Leinonen V, Vähä-Koskela M, Zhang Q, Kühnel F, Mei Y-F, Ylä-Herttuala S, Jääskeläinen JE, Hinkkanen A: Immunohistochemical characterization and sensitivity to human adenovirus serotypes 3, 5 and 11p of new cell lines derived from human diffuse grade lI-IV grade gliomas. Transl Oncol., 10: 772-779, 2017
-
 Viral vectors are powerful gene therapy and research tools. To improve them we have been developing different strategies to either target them to specific cell types or to use them to regulate endogenous gene expression. These strategies have in common our use of either non-coding RNA or elements of the non-coding genome, such as super-enhancers. Endothelial cells are the sole building blocks of blood vessels and so targeting therapeutic vectors to these cells would greatly benefit angiogenic gene therapy. To achieve this, we have screened super-enhancers, which have been associated with cell lineage, to select from within these enhancers to limit expression to endothelial cells. We successfully identified an enhancer that allows expression of a vector transgene specifically to endothelial cells and by using CRISPR/Cas9 deletions of these enhancers we were able to identify previously unknown mediators of vessel sprouting. Our rationale strategy has yielded targeted vectors and is a proof-of-principle for development of other cell-type vectors.
Viral vectors are powerful gene therapy and research tools. To improve them we have been developing different strategies to either target them to specific cell types or to use them to regulate endogenous gene expression. These strategies have in common our use of either non-coding RNA or elements of the non-coding genome, such as super-enhancers. Endothelial cells are the sole building blocks of blood vessels and so targeting therapeutic vectors to these cells would greatly benefit angiogenic gene therapy. To achieve this, we have screened super-enhancers, which have been associated with cell lineage, to select from within these enhancers to limit expression to endothelial cells. We successfully identified an enhancer that allows expression of a vector transgene specifically to endothelial cells and by using CRISPR/Cas9 deletions of these enhancers we were able to identify previously unknown mediators of vessel sprouting. Our rationale strategy has yielded targeted vectors and is a proof-of-principle for development of other cell-type vectors.Our future studies include identification of endothelial and hypoxia specific circular RNA, another class of non-coding RNA that have recently been identified that may be important in gene regulation. These may offer further avenues for gene therapy.
People involved
- Isidore Mushimiyimana, Mustafa Beter, Tuisku Suoranta, Maria Barbiera, Hassan Sghayyar, Nihay Laham-Karam, Seppo Ylä-Herttuala
Collaborators
Selected publications
- Mushimiyimana I, Niskanen H, Beter M, Laakkonen JP, Kaikkonen M, Ylä-Herttuala S., Laham-Karam N. Discovery and characterisation of functional endothelial super-enhancers. Nucleic Acids Research. 2021; 20;49(14):8078-8096.
- Mushimiyimana I, Tomas Bosch V, Niskanen H, Downes N, Ylä-Herttuala S, Laham-Karam N, Kaikkonen M. Genomic landscapes of VEGF family members in endothelial cells. Molecular and Cellular Biology 41(7): e0059420.
- Suoranta T, Laham-Karam N, Ylä-Herttuala S. Optimised protocol for accurate titration of AAV vectors. Human Gene Therapy. 2021;32(19-20).
-
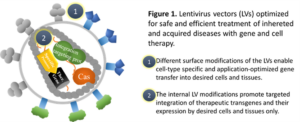 Our research interests cover the development of safe and efficient gene transfer tools and their use to treat inherited and acquired diseases, such as familial hypercholesterolemia and heart failure with gene therapy. We also aim to generate safe and maximally efficient chimeric antigen receptor T cells (CAR T cells) for the needs of cell therapy.
Our research interests cover the development of safe and efficient gene transfer tools and their use to treat inherited and acquired diseases, such as familial hypercholesterolemia and heart failure with gene therapy. We also aim to generate safe and maximally efficient chimeric antigen receptor T cells (CAR T cells) for the needs of cell therapy.Our primary tools are lentivirus vectors (LVs), which we have optimized at many levels to be best suited for disease-specific treatments. Surface-modifications of the vectors are designed to limit gene transfer to desired cells and tissues only, while targeted integration of the therapeutic genes into genomic safe harbor sites minimizes the risks for insertional mutagenesis. These modifications reduce potential side-effects of gene therapy, minimize the risks for adverse events and improve the overall safety of gene therapy. In addition to LVs that carry modifications in their integration machinery, we use the CRISPR/Cas system for targeted integration and genome editing.
Our most recent publication shows how the introduction of an endonuclease into the integration machinery of LVs efficiently retargets vector integration away from unique protein-encoding genes and promotes targeted integration into the area of interest with an over 20% efficiency (Schenkwein et al, 2020).
People involved
Collaborators
Selected publications
- Schenkwein D, Afzal S, Nousiainen A, Schmidt M, Ylä-Herttuala S. Efficient Nuclease-Directed Integration of Lentivirus Vectors into the Human Ribosomal DNA Locus. Mol Ther. 2020 Aug 5;28(8):1858-1875. doi: 10.1016/j.ymthe.2020.05.019.
-
 The mouse has been an important model organism to study human cardiovascular diseases due to its relatively high genome similarity and fast breeding rate. Our mouse models cover many cardiovascular diseases such as atherosclerosis, heart failure and ischemic heart disease. In addition to these, we use small in vivo models to study lipid metabolism, type 2 diabetes related cardiovascular complications and ocular neovascularisation. Our goal is to find novel gene therapies for these devastating diseases as well as to study the disease pathology.
The mouse has been an important model organism to study human cardiovascular diseases due to its relatively high genome similarity and fast breeding rate. Our mouse models cover many cardiovascular diseases such as atherosclerosis, heart failure and ischemic heart disease. In addition to these, we use small in vivo models to study lipid metabolism, type 2 diabetes related cardiovascular complications and ocular neovascularisation. Our goal is to find novel gene therapies for these devastating diseases as well as to study the disease pathology.People involved
- Sanna Kettunen, Erika Gurzeler, Annakaisa Tirronen, Rahul Mallick, Sanna Koponen, Krista Hokkanen, Galina Wirth, Diana Törmä, Anna Slita, Durga Devi Thota, Anna-Kaisa Ruotsalainen and Seppo Ylä-Herttuala
Selected publications
- Gurzeler E, Aavik E, Laine A, Valkama T, Niskanen H, Huusko J, Kaikkonen MU, Ylä-Herttuala S. Therapeutic effects of rosuvastatin in hypercholesterolemic prediabetic mice in the absence on low density lipoprotein receptor. Biochim Biophys Acta Gen Sub. 2019 Feb;1863(2):481-490.
- Tirronen A, Vuorio T, Kettunen S, Hokkanen K, Ramms B, Niskanen H, Laakso H, Kaikkonen MU, Jauhiainen M, Gordts PLSM, Ylä-Herttuala S. Deletion of lymphangiogenic and angiogenic growth factor VEGF-D leads to severe hyperlipidemia and delayed clearance of chylomicron remnants. Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2327-2337.
- Kokki E, Karttunen T, Olsson V, Kinnunen K, Ylä-Herttuala S. Human vascular endothelial growth factor A(165) expression induces the mouse model of neovascular age-related macular degeneration. Genes (Basel). 2018 Aug 31;9(9):438.
- Devi D, Babu M, Mäkinen P, Kaikkonen MU, Heinäniemi M, Laakso H, Ylä-Herttuala E, Rieppo L, Liimatainen T, Naumenko N, Tavi P, Ylä-Herttuala S. Aggravated Postinfarct Heart Failure in Type 2 Diabetes Is Associated with Impaired Mitophagy and Exaggerated Inflammasome Activation. Am J Pathol. 2017 Dec;187(12):2659-2673.
-
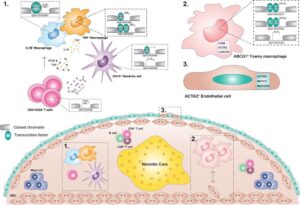
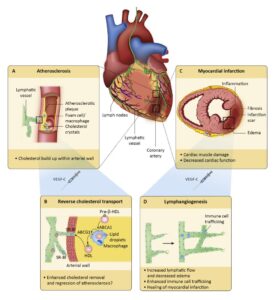
Human atherosclerotic plaque microanatamical characterisation using single-cell transcriptomics (Depuydt et al 2020) Histopathological analysis alongside with sophisticated imaging techniques (MRI, IVUS, CT etc.) have been used to look for changes in arterial wall that will explain the atherosclerotic lesion formation. Histopathological methods reach single cell resolution but are limited to a small number of markers detectable per tissue slice at a time. This small number of markers (3 – 5 proteins and/or mRNAs) does not allow to distinguish between the subtleties in phenotypes of cells communicating in different types of atherosclerotic lesions. Imaging techniques (MRI, IVUS, CT) are non-invasive, but their spatial resolution is too low to detect individual cells. Collectively, our knowledge about the fine structure (microanatomy) of atherosclerotic lesions needs to be elaborated with the help of modern techniques.
Modern massively parallel single-cell RNA sequencing (scRNA-seq) allows specification of sets of hundreds of transcripts associated with individual cells, but information regarding the positions of these cells within tissue gets lost upon tissue dissociation. Spatial transcriptomics techniques, on the contrary, map the location of transcripts in tissue precisely but fail to define the identity of the cells that expressed these transcripts. Our aim is to make the most out of the complementary strengths of our scRNA-seq data and advanced spatial transcriptomics technique in analyzing the microanatomy of atherosclerotic lesions. Combining data from the two techniques will allow us to demarcate the biological identity and spatial distribution of cells comprising the architecture of different types of atherosclerotic lesions.
In a recent publication (Depuydt et al 2020) we have determined the molecular characteristics of 14 distinct cell types present in human atherosclerotic lesions by scRNA-seq analysis. Molecular profiling of thousands of individual cells has made it possible to pre-select markers suitable for mapping and quantitating defined cell types communicating with each other in different types of atherosclerotic lesions. Expansion microscopy is a novel approach in spatial transcriptomics and it allows detection of tens or even hundreds of expressed genes simultaneously in a tissue block without need for super-resolution microscope.
People involved
Collaborators
- Tiit Örd (UEF)
Selected publications
- Depuydt et al. 2020. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics.
-
Our research focuses on defining disease mechanisms and developing therapy for cardiovascular diseases. We use state-of-the art cell biological, imaging and sequencing methods to study clinical samples, and identify novel mediators that are used for gene therapy to modulate vascular growth. Our recent publication demonstrated for the first time that BMP6, a growth factor, is able to induce vascular growth and regulate VEGFR2/Hippo signaling, and thus can act as a potential therapeutic target.People involved
- Henna Ilmonen, Miika Kiema, Emma Luoto, Heidi Pulkkinen, Heta Rasinkangas, Suvi Jauhiainen, Johanna Laakkonen
Select Publications
- Pulkkinen HH, Kiema M, Lappalainen JP, Toropainen A, Beter M, Tirronen A, Holappa L, Niskanen H, Kaikkonen MU, Ylä-Herttuala S, Laakkonen JP. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis. 2020; 24: 129–144.
Funding
Collaborators
-
- Mauro Giacca, Director-General ICGEB Group Leader
- Thierry Vanden Driessche, Professor in medicine
- Marinee Chuah, Associate Professor, Vrije Universiteit Brussel
- Thomas Thum, Professor of Cardiology, Imperial College London
- Juha Hartikainen, Professor of cardiology, University of Eastern Finland
- Reinier A. Boon, University of Frankfurt
- Andrew Baker, Professor of Translational Cardiovascular Sciences, University of Edinburgh
Leaders
Senior Researchers
-

Nihay Laham Karam
Research DirectorA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Johanna Laakkonen
Academy Research FellowA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Petri Mäkinen
Staff ScientistA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Minna Niittykoski
Project ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Diana Schenkwein
Senior ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Suvi Jauhiainen
Project ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Jarkko Hytönen
Senior ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences
Post-doctoral Researchers
-

Anna-Kaisa Ruotsalainen
Postdoctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Erika-Nina Gurzeler-Tiihonen
Research ManagerA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Venla Olsson
Postdoctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Isidore Mushimiyimana
Postdoctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Alisa Nousiainen
Postdoctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences
Doctoral Researchers
-
Maria Barbiera
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Mustafa Beter
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Theodor Boc
Doctoral StudentA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Pracheta De
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Minja Heikkilä
Doctoral StudentA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Krista Hokkanen
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Sanna Kettunen
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Miika Kiema
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Riku Kiviluoto
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Sanna Koponen
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Jaakko Lampela
Doctoral StudentA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Rahul Mallick
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Juho Pajula
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Heidi Pulkkinen
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Iida Räty
Doctoral Researcher -
Anna Slita
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Aino Sormunen
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -

Tuisku Suoranta
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Diana Törmä
Doctoral ResearcherA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Hanna Wang
Doctoral StudentA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Galina Wirth
Visiting Researcher
Technicians
-
Sari Järveläinen
Senior Laboratory TechnicianA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Tiina Koponen
Senior Laboratory TechnicianA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Eila Korhonen
Laboratory TechnicianA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Maarit Mähönen
Laboratory TechnicianA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences -
Sonja Kotoneva
Laboratory TechnicianA.I. Virtanen Institute for Molecular Sciences, Faculty of Health Sciences


















